Stimuli-Responsive Functional Materials
The central theme of our research endeavors is to develop smart functional materials that interact with and respond to various chemical and physical stimuli, such as guest molecules and ions, applied electric field, and light, enabling anion recognition and sensing, light-to-electrical energy conversion, charge conduction, energy storage, and pH-controllable guest uptake and release, among other advanced applications. We exploit synthetic chemistry to prepare structurally and functionally encoded molecular building blocks, molecular self-assembly to assemble these building blocks into nanoscale materials, and a range of spectroscopic, analytical, and physical techniques to explore their properties and functions. Our multifaceted research projects seek to unveil fundamental knowledge in materials and supramolecular chemistry and apply this knowledge to address key challenges in the following areas:
Electronic and Photonic Metal–Organic Frameworks (MOFs)
Composed of metal ion clusters acting as nodes and organic ligands as beams, pillars, walls, and floors, hybrid porous MOFs emerged as one of the most fascinating and versatile functional materials of this century. Although their separation, storage, and delivery capabilities are well-documented, electronic and photonic MOFs capable of solar energy conversion, charge transport, and storage are few and far between, chiefly due to the scarcity of redox and photoactive building blocks, difficulties of integrating them into devices, and the lack of intrinsic charge transport pathways within these porous materials. To address these challenges and transform MOFs into next-generation electronic and photonic materials, w
e are constructing stimuli-responsive MOFs using electronically and optically active ligands, growing robust, oriented MOF films on metal oxide substrates that can be easily integrated into devices, and investigating how they adapt to guest molecules and ions, applied electric field, and light. The guest-induced color and conductivity changes lead to sensing, while those triggered by electric field and light would expand their utility in electronic devices, batteries, solar cells, and energy efficient lights. We have demonstrated that intercalation of complementary guest π-systems between the electroactive MOF ligands lowers their band gaps (ACS-Appl. Mater. Interfaces 2017) and enhances their electrical conductivity (J. Mater. Chem. C 2016).
Anion and Ion-Pair Recognition and Sensing with π-Acidic Receptors
Anions play important roles in myriads of biological, chemical, agricultural, and industrial processes, making precise manipulation of anions an important and active research area of supramolecular chemistry. While traditional hydrogen-bonded anion receptors are able to differentiate anions on the basis of their size and shape, they cannot distinguish anions the basis of their intrinsic electronic properties, i.e., charge density, Lewis basicity, and reducing power. Adding a new dimension to anion recognition chemistry, we discovered that π-acidic receptors, such as of naphthalenediimides (NDIs) and perylene diimides (PDIs) discriminate anions on the basis of their electronic properties by undergoing ground state electron transfer from strong Lewis basic anions (e.g., fluoride, hydroxide) and photoinduced electron transfer from less Lewis basic anions (e.g., chloride, acetate), which reduce them to paramagnetic radical anions and occasionally dianions (
JACS 2010,
2011,
2012;
OrgBiomolChem. 2013), while forming charge transfer complexes with non-Lewis basic anions (e.g., bromide, iodide) and anion–π complexes with multinuclear charge diffuse anions (e.g., triflate, perchlorate) (
CrystEngComm 2012;
ChemComm 2013).
We then expanded the anion-induced electron transfer phenomenon to Lewis acids, demonstrating that in aprotic solvents fluoride reduces silver(I) cation to silver(0) generating fluorescent silver clusters / nanoparticles in solutions and metallic silver films on glass surfaces (JACS 2015). With the help of NSF support, we are currently developing NDI-based novel ion-pair receptors that not only capture anions and cation simultaneously in a cooperative fashion, but also release them upon electrochemical or photochemical inputs. We are particularly interested in regulating charge-diffuse anions, such as perchlorate (explosive), pertechnetate (radioactive waste), and perrheneate (nuclear medicine), which are difficult to capture with traditional hydrogen bonded receptors, but prefer receptors with soft, hydrophobic binding sites.
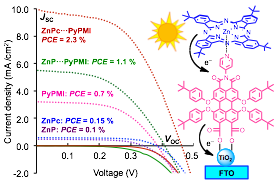
Multichromophoric Supramolecular Solar Cells
The prospect of addressing skyrocketing energy demand to power and advance modern technologies in a clean, safe, and sustainable way depends in part on the availability of efficient light-harvesting materials that can convert light to electrical energy throughout the visible–near-IR range. Dye-sensitized solar cells made of single chromophore / electron donor units typically suffer from poor photoconversion efficiency due to (i) narrow light absorption window and (ii) poor charge separation and rapid charge recombination. To addressed these issues, we are constructing multichromophoric dye-sensitized solar cells based on coordinatively linked chromophores, electron donors, and acceptors, such as Zn-porphyrin, Zn-phthalocyanine, and perylene dyes (ChemComm 2012, 2014
) that display notably higher energy conversion efficiencies than those made of individual dyes. Building on this knowledge, we are now developing light-harvesting metal-organic frameworks for solar energy conversion.
pH-Responsive Vesicles and Nanotubes Based on Amphiphilic Macrocycles
For controlled delivery of drugs and nutrients to certain parts of our bodies, we need nanoscale capsules and vesicles that can encapsulate these guest molecules, traverse through cell membranes, and release molecular cargo inside certain target cells when activated by chemical or physical inputs. To this end, we have designed and synthesized two amphiphilic hexaamide macrocycles via template-directed macrocyclization (Chem Comm 2013
). In neutral polar solvents, the nitro-functionalized macrocycle self-assembled into closed vesicles (~100 nm diameter), while the amine-functionlaized macrocycle formed partially open vesicles or bowls. Upon acidification of the medium, both spherical nanostructures morphed into nanotubes and fibers, as observed through electron microscopy. We envision that these pH-responsive vesicles could potentially serve as controlled drug-delivery systems that could release encapsulated drug molecules inside cancer cells, which are usually more acidic than the normal healthy cells.